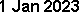Putting Glass Walls on New York's Slaughterhouses So We Can
See Behind Closed Doors
Gold Medal Packing
Address: 8269 River Rd, Rome, NY 13424
Establishment No.: m17965
USDA Inspection Report: 19 Jun 2012
Code: 03C02
Violation: 304.3(c), 310.22(a)(1),
310.22(e)(1), 310.22(g)(2), 417.2(c)(5), 417.2(c)(7), 417.2(c)(7),
417.2(d), 417.2(e)
Citation: On June 20, 2012, I was reviewing Gold Medal"s new processing steps in HACCP Plan for incoming beef over 30 months of age. The following non compliance is in the receiving step for beef from New York Custom in the newly addressed V. Hazard Analysis in HACCP Plan for Raw, Not Ground Products in Tact.In Gold Medal's HACCP Plan for [redacted] is in place, but it only addresses beef under the age of 30 months. The establishment does not define how they are going to lot or test the beef carcasses ( over 30 months of age received from New York Custom Processors) for E. coli 0157:H7. At first, stated that they didn't need testing because NYC does the testing. said that they would be testing each lot.[newline]Two bins of boneless trimmings were tagged with US Retained tag 39320082 and 3920083. A pallet with 18 boxes containing 2 boxes of Beef Tenderloin, 4 boxes of Beef Knuckles, 7 boxes of Beef Top Round and 5 boxes of Ribeye was retained with US Retained tag # 39320084. and were informed of the situation.
Regulation:
304.3(c) Before producing new product for distribution in commerce, an establishment shall have conducted a hazard analysis and developed a HACCP plan applicable to that product in accordance with § 417.2 of this chapter. During a period not to exceed 90 days after the date the new product is produced for distribution in commerce, the establishment shall validate its HACCP plan, in accordance with § 417.4 of this chapter.
310.22(a)(1) The brain, skull, eyes, trigeminal ganglia, spinal cord, vertebral column (excluding the vertebrae of the tail, the transverse processes of the thoracic and lumbar vertebrae, and the wings of the sacrum), and dorsal root ganglia from cattle 30 months of age and older and
310.22(e)(1) Establishments that slaughter cattle and establishments that process the carcasses or parts of cattle must develop, implement, and maintain written procedures for the removal, segregation, and disposition of specified risk materials. These procedures must address potential contamination of edible materials with specified risk materials before, during, and after entry into the establishment. Establishments must incorporate their procedures for the removal, segregation, and disposition of specified risk materials into their HACCP plans or Sanitation SOPs or other prerequisite programs.
310.22(g)(2) Ensures that the carcasses or parts are accompanied by documentation that clearly states that the carcasses or parts contain vertebral columns from cattle that were 30 months of age and older at the time of slaughter;
417.2(c)(5) Include all corrective actions that have been developed in accordance with § 417.3(a) of this part, to be followed in response to any deviation from a critical limit at a critical control point; and
417.2(c)(7) List the verification procedures, and the frequency with which those procedures will be performed, that the establishment will use in accordance with § 417.4 of this part.
417.2(d)(1) The HACCP plan shall be signed and dated by the responsible establishment individual. This signature shall signify that the establishment accepts and will implement the HACCP plan.
417.2(e) Pursuant to 21 U.S.C. 456, 463, 608, and 621, the failure of an establishment to develop and implement a HACCP plan that complies with this section, or to operate in accordance with the requirements of this part, may render the products produced under those conditions adulterated.
Next Report: USDA Inspection Report: 20 Jun 2012
Previous Report: USDA Inspection Report: 5 Jun 2012
Return to:
Gold Medal Packing
Return to: New York Slaughterhouses